|
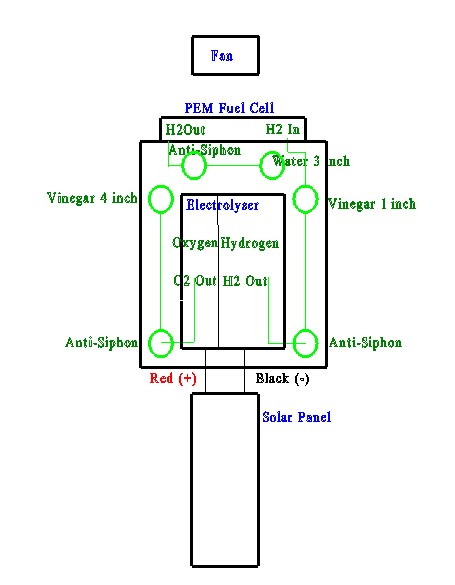
System Functional Description Solar Panel: The solar panel converts sunlight into electrical energy by the excitation of silicon.
Electrolyser: The electrolyser splits water into hydrogen and oxygen. Pure water is an insulator, so an electrolytic solution (25% KOH) is needed to make the water conductive. The KOH is constantly split by the electrical flow, reacts with the water, and produces free H2 and O2. Hydrogen can be thought of as a "molecular" battery, each hydrogen atom is storing 1 electron that can be used to produce electrical current later.
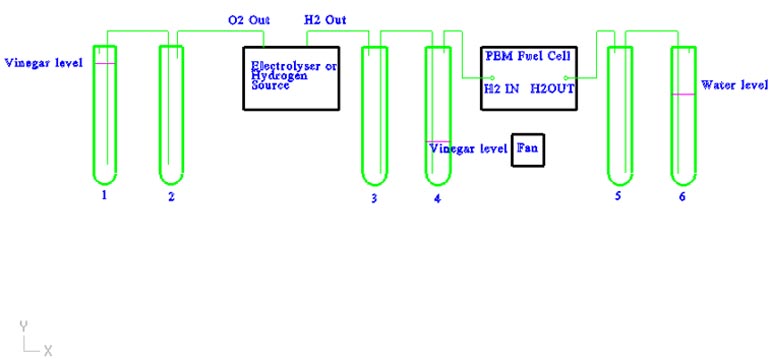
Scrubbing test tubes(1-4): The output hydrogen and oxygen are fed through a 5% red wine vinegar to nuetralize the KOH. The vinegar on the oxygen side is filled to provide backpressure. The vinegar on the hydrogen side is low, allowing the backpressure to be created after the fuel cell.
Each test tube has an anti-siphon test tube (2-3) to prevent vinegar from getting into the electrolyser.
PEM Fuel Cell: The PEM fuel cell is the "engine" that will take the hydrogen and combine it with oxygen from the air. The Proton Exchange Membrane and the bipolar plates provide a current path for the electrons (50-60% of them) that are given off by the hydrogen atom when it combines with oxygen to form water. Nominal efficiency of a fuel cell is around 50%-60%, so the additional electrons are converted into heat energy.
Backpressure test tubes (5-6): These test tubes keep a backpressure on the fuel cell that can be directly measured in inches/water column. The water also acts as a trap to keep air out of the fuel cell. The anti-siphon test tube (5) keeps the water from being pulled into the fuel cell.
|



