How an electrolyser works An electrolyser seperates the water into hydrogen and oxygen by splitting the molecules with an electrical current. The elctrical current forces two electrons to attach to the hydrogen atoms, splitting it from the oxygen. The process is not efficient, and is not the most economical way to produce hydrogen, but is the most convenient. |
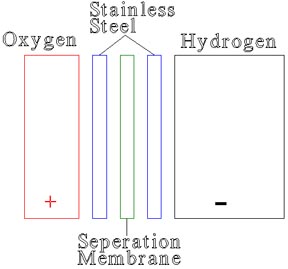
The oxygen cavity contains the KOH and water used for the electrolytic solution. The KOH is used because it will continue in a chain reaction when driven by an electrical current, splitting from the OH, and immediately attaching to to another OH. As long as an electrical current is supplied, and water is available the unit will produce hydrogen.
The stainless steel plates provide the surface area for the electrolyser. An electrolyser is dependent on surface area, proximity of plates, electrolytic solution, voltage and current. The proper voltage is 2-3 volts DC.
The seperation membrane provides a mechanical seperation between the hydrogen and oxygen, and allows the current to flow between the steel plates.
The hydrogen cavity is sized twice as large as the oxygen cavity, and is the main by-product of the electrolyser for use in the fuel cell. For an industrial system a large scale electrolyser would be used and the resultant gasses compressed for later use, or used directly for manufacturing. |



